The mouse model
You may have heard that the new COVID-19 booster shots were largely evaluated on mouse data.1
Is this the new normal? Are we going to rely more on mice experiments for future “updates” to COVID-19 vaccines?
If that’s the case, it’s worth asking: is there any evidence that mice can handle the toxicity of mRNA vaccines better than humans?
Unfortunately, there is.
Humans are more sensitive than mice to the same dose of mRNA vaccine
We’ll focus on this fascinating paper from March 2022. It’s worth going over in some detail.
This study looked at how mice, humans, and cell cultures reacted to various lipid-formulated mRNA vaccines. They focused on the short term effects of the vaccines; specifically the effects of the mRNA itself, or the lipids used to encase the mRNA, as opposed to the proteins that were encoded by the mRNA.
We’ll first look at their results with a vaccine they call “RNA-LPX.” This is an mRNA cancer vaccine that’s supposed to stimulate T cells to recognize neoantigens, which are proteins specifically expressed in tumor cells. The mRNA is encased in a “lipoplex,” which is a positively charged liposome2 that’s a bit different than the lipid nanoparticles (LNPs) used in the COVID-19 vaccines.
We’ll get to their results with LNP-formulated mRNA vaccines later.
Anyway, they found that mice were less sensitive to these vaccines compared to humans:
In contrast to humans, C57BL/6 and Balb/c mice are remarkably tolerant to RNA vaccines and only display limited systemic cytokine release following i.v. administration of a liposomal vaccine containing unmodified RNA (RNA-LPX).
“C57BL/6” and “Balb/c” are different strains of lab mice.
The same dose of mRNA vaccine was “well tolerated” in mice, but led to flu-like symptoms in humans, despite the vast difference in size:
Even at doses of RNA (50 µg) that are well tolerated in mice, patients exhibit transient mild-to-moderate flu-like symptoms... Given the obvious size differences, this means that RNA-LPX doses that trigger potent systemic inflammatory responses in humans are more than 1,000-fold lower than in inbred laboratory mice.
When the COVID-19 vaccines were being rolled out, some people would complain of extreme fever, chills, aches or fatigue. Remember what we were told about that back then? It means it’s “working.”3
Well, they were probably also causing high levels of systemic inflammation.
For reasons we’ll get into later, mice don’t respond quite the same way. In fact, doubling that dosage was fine in mice: “high-dose RNA-LPX (100 μg) was well tolerated in wildtype mice without any detectable adverse events.”
The reason for these differences is not known:
Similar observations have been made with other pro-inflammatory stimuli, creating a notable discrepancy in the dose needed to induce biological and toxicological responses in different species. The mechanisms underlying these dramatic differences have remained largely unknown.
The effect may in part have to do with the levels of particular cytokines
Part of the difference in response may have to do with differing levels of certain cytokines, which are small proteins that are used to communicate between cells.
In particular, in response to these vaccines, humans seem to produce more “pro-inflammatory” cytokines.
Pro-inflammatory cytokines are correlated with higher inflammation, and also cause a rise in other pro-inflammatory cytokines, which in turn can cause even more inflammation. Although inflammatory cytokines are a necessary part of defense against pathogens, high systemic levels can lead to harmful, out-of-control immune responses.
We uncovered the key role of IL-1 in triggering the release of other pro-inflammatory cytokines associated with cytokine release syndrome (CRS), with humans being markedly more sensitive than mice.
Moreover, in response to these vaccines, murine leukocytes (mouse white blood cells), release more anti-inflammatory cytokines, which serve to dampen inflammation:
Unlike humans, murine leukocytes respond to RNA vaccines by upregulating anti-inflammatory IL-1ra relative to IL-1… protecting mice from cytokine-mediated toxicities at >1,000-fold higher vaccine doses.
The figure above shows the levels of systemic IL-1 type cytokines over predose levels after 25-μg RNA-LPX administration in 9 humans and 8 mice. The humans here were cancer patients participating in a phase 1b study (NCT03289962) for this mRNA vaccine.
Note that the mice were administered identical absolute amounts as the humans.
Mice seem to release a lot more of the anti-inflammatory IL-1ra, compared to the inflammatory IL-1 cytokines (which come in two forms, IL-1β and IL-1α).
But what if this effect just has to do with the fact that the humans in this experiment had cancer?
Well, we also see this pattern when we compare healthy human and mouse cells in vitro:

In the figure above, we see results from (a) human PBMCs (human peripheral blood mononuclear cells) donated from healthy human volunteers, and (b) mouse blood cells.
In the human cells, inflammatory IL-1β (red) increased and greatly exceeded anti-inflammatory IL-1ra (green) at higher doses of the RNA-LPX vaccine.
In mouse blood cells, the anti-inflammatory IL-1ra was already high at baseline, and further increased with higher RNA-LPX dose, while the inflammatory cytokines were only really observed at high dose levels.
This was seen in lab mice regardless of vendor origin or strain (Extended Data Fig. 5c).
Notice that the scales are different in these graphs; if they’d had the same scale, the differences between human and mouse would be even more stark.
Increases in other inflammatory cytokines, like IL-6 and TNF-α, were also much higher in human blood cells compared to mouse blood cells (Extended Data Fig. 5b).
Mice deficient in IL-1ra (like humans) respond worse than normal mice
So mice release more IL-1ra compared to humans, and we think that this helps to dampen inflammation in mice. But do high levels of IL-1ra really dampen inflammation, or is this just a correlation we’re seeing?
In order to answer that question, the researchers tested RNA-LPX on IL-1ra-deficient (Il1rn−/−) mice. In contrast to normal (“wildtype”) mice:
RNA-LPX-treated Il1rn−/− mice rapidly developed a CRS-like phenotype characterized by pronounced hypothermia (Fig. 3e), body weight loss (Fig. 3f) and excessive systemic cytokine release (Fig. 3g).
These mice appeared to suffer from more “CRS,” which stands for “cytokine release syndrome.” On the other hand, “high endogenous levels of IL-1ra can protect wildtype mice from immune dysregulation and uncontrolled systemic inflammation.”
IL-1ra-deficient mice may be more representative of how humans react to these kinds of vaccines:
IL-1ra-deficient mice can better predict patient responses to innate immune challenges (such as RNA vaccines) and provide a useful tool to evaluate both the sensitivity to pathogens and tolerability to treatment-related inflammatory toxicities in vivo.
Moreover, IL-1ra-deficient mice were more sensitive to other toxins as well, including toxins from bacteria. Fascinating.
It’s not just this mRNA vaccine
Maybe it’s just this particular mRNA vaccine that’s particularly inflammatory?
That doesn’t seem to be the case.
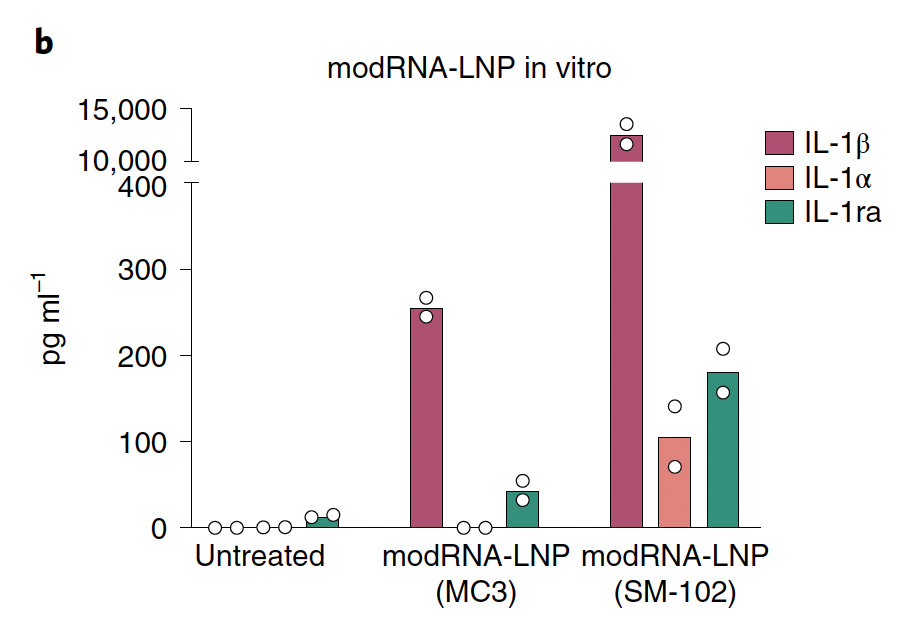
This study also generated mRNA vaccines that encoded for eGFP, which is a fluorescent protein.4 They made several versions using different lipid formulations to encase the mRNA in.
One version used liposomes like the RNA-LPX discussed above, and two used LNPs formulated with different ionizable lipids; one using SM-102, which was used in the Moderna COVID-19 mRNA vaccine, and the other using MC3, which has been used for other RNA therapeutics.
Of all the vaccines, the one with SM-102 was most inflammatory, though both LNP-formulated vaccines caused a marked increase in the inflammatory cytokine IL-1β in human PBMCs, while the levels of anti-inflammatory IL-1ra were lower:
Which components of mRNA vaccines are inflammatory?
For some mRNA vaccines, both the mRNA and lipid component have to be present in order to cause inflammatory cytokine release, whereas in others, the lipid alone is sufficient. It depends on the specific lipid formulation, and whether the mRNA is “modified.”
Modifying mRNA, where the uridines are replaced with N1-methyl-pseudouridine, as was done with the COVID-19 mRNA vaccines, allows the mRNA to evade certain toll-like receptors within cells, which are proteins that detect single stranded RNA like certain types of viral RNA. mRNA vaccines that contain unmodified mRNA will stimulate these TLRs and make the cells “think” that there’s been a viral invasion. This is one of the signals that can contribute to the release of inflammatory cytokines.
The image above shows levels of cytokines with different mRNA vaccines in human PBMCs. Wherever you see “mod” it means that the mRNA was modified.
We see that mRNA with the lipoplex lipid (RNA-LPX) leads to the release of lots of inflammatory cytokines, whereas modified mRNA with this same lipid (modRNA-LPX) does not.
That indicates that the lipoplex by itself does not cause the release of inflammatory cytokines, and indeed when they tested just the lipid alone without any mRNA, it didn’t lead to release of any inflammatory IL-1β (Fig. 1f).
The LNP lipid used in the Moderna mRNA vaccine is inflammatory on its own
On the other hand, modRNA-LNP(SM-102), which uses the Moderna lipid, is highly inflammatory despite the fact that it has modified RNA. In fact, they saw potent release of inflammatory IL-1β even with empty LNP with SM-102 (Fig. 7c).5
Surprisingly, the reactogenicity of RNA vaccines was not necessarily due to the TLR7/8 agonism, as IL-1 release was observed using vaccines containing N1-methyl-pseudouridine-modified RNA (modRNA). Instead, the lipid components used to formulate these vaccines substituted for unmodified RNA in eliciting the IL-1 response.
“TLR7/8 agonism” means stimulation of toll-like receptors 7 and 8 (which recognize single stranded RNA in cells).
This may not be surprising given that there have been other papers pointing to the inflammatory nature of some of these LNPs (see here or here).
Unfortunately, this study didn’t test the ionizable lipid that was used in the BioNTech/Pfizer mRNA vaccine, ALC-0315.
They also only tested the Moderna-like modRNA-LNP(SM-102) vaccine on human cells so we can’t compare the results with mouse cells. My guess is that mice would respond better to it than humans, just like with RNA-LPX, but this would obviously need to be tested.
Multiple sources of toxicity in the COVID-19 mRNA vaccines
How can we relate these findings to what we know about the COVID-19 mRNA vaccines?
Well we know that the COVID vaccines encode for the SARS-CoV-2 spike protein, which appears to be harmful to cells. So we now have published evidence that COVID-19 mRNA vaccination can lead to at least two sources of toxicity: the LNPs, at least in the case for Moderna, and spike protein.
My guess is that the most acute effects of LNPs and spike protein are elicited at different times post-vaccination. I would expect that the most obvious effects from the lipids occur soon after vaccination, whereas the most obvious effects from spike protein occur a bit later, after cells have had enough time to produce enough spike protein.
There could also be longer term or more chronic effects from either.
Nonhuman primate cells
This study also tested how some nonhuman primate cells responded to RNA-LPX vaccine:
We also measured the respective cytokine levels in nonhuman primate (NHP) cells, as cynomolgus macaques and rhesus macaques are often used to assess safety and immunogenicity of RNA vaccines. Interestingly, robust upregulation of IL-1ra was detected at all RNA-LPX dose levels, while IL-1β concentration and monocyte frequency were found to be lower in both cynomolgus macaque and rhesus macaque PBMCs compared with human PBMCs (Extended Data Fig. 5d–f).
In other words, the nonhuman primate cells upregulated the anti-inflammatory cytokines in relation to the inflammatory cytokines.
Our studies with NHP PBMCs also suggest that NHPs resemble mice more than humans with respect to their monocyte frequencies and IL-1ra induction profile.
And:
These results suggest that similar to mice, preclinical studies in NHPs might not fully capture the inflammatory toxicities related to RNA vaccines.
So using other primates to test these vaccines on may also be problematic.
Lab mice compared to wild mice
As mentioned earlier, this study looked at different substrains of lab mice.
This was a good thing, because substrains of lab mice can be noticeably different. This study looked at two closely related substrains of mice from different vendors, and found differences in size, sociability, responses to stress, circadian activity, and proclivity to weight gain under stress (we’ve all been there).
Given that even substrains of lab mice can vary so much, it’s worth asking: what would the results of this study have been if they’d also looked at wild mice?
Bret Weinstein, an evolutionary biologist, has talked about how breeding protocols for lab mice have led to major differences between wild and lab mice. This has led to lab mice having longer telomeres, which would endow them with greater capacity for tissue repair, though this would be at the expense of being able to control tumors (for more, see here or here).
This could obviously have enormous ramifications for drug or vaccine safety testing.
Much more can be said about this, but for now it’s something to keep in mind as our regulatory agencies rely heavily on lab mouse data to influence what “therapeutics” get approved for humans.
Main takeaways
To sum up:
The same dose of an mRNA formulated with lipoplex (cationic liposome) vaccine led to flu-like symptoms in humans but not in mice, despite the large size difference.
That vaccine induced more systemic inflammatory cytokine release in humans compared to mice.
That vaccine also led to less anti-inflammatory cytokine release in humans, compared to mice.
This cytokine pattern was also seen when comparing human blood cells with mouse blood cells in vitro.
The pattern was also seen across several different strains of lab mice.
The pattern was also seen in nonhuman primate cells in vitro.
Modifying the mRNA in the mRNA vaccine formulated with lipoplex does not induce inflammatory cytokines in human blood cells.
mRNA vaccines formulated with LNPs induced inflammatory cytokines in human blood cells, despite mRNA modification.
The LNP formulated with SM-102, which was used in the Moderna COVID-19 mRNA vaccine, was the most inflammatory out of the different lipid formulations tested.
Empty LNP with SM-102 by itself was inflammatory.
Lastly, it should be mentioned that none of what was in this article is to say that mice are worse than other animal models; after all, we saw that nonhuman primate cells reacted similarly to mouse cells.
But I hope most of us can agree that relying heavily on mouse data is highly inappropriate for authorizing products that will be given to perfectly healthy people, including children.
You may hear factcheckers say that this claim is “misleading” because part of the FDA’s decision to give authorization for this booster was also based on a small human trial from a “similar booster” that targeted BA.1. You can decide for yourself whether that was appropriate or good enough, but it should be made clear that the booster that was authorized was a different product; the authorized booster is a bivalent mRNA vaccine that has mRNA from the original strain plus the BA.4 and BA.5 omicron subvariants.
You’ll also see this being referred to as a cationic liposome.
There’s a fine balance that vaccines need to play: on the one hand they need to stimulate components of the innate immune system (which releases inflammatory cytokines) in order to get the attention of the adaptive immune system (B and T cells), but obviously too much inflammation is bad. The ideal vaccine would trigger just enough that is necessary.
This protein is frequently chosen for all kinds of experimental settings. For one, it’s easy to see whether it’s being expressed in cells.
In other words, this lipid actually seems to act as an adjuvant.






Joomi, please stop being so clear and intelligent. You are making the big pharma companies, the CDC and the FDA look stupid or malevolent or both.
Thanks for the article. I hadn't noticed the paper and will have to give it a read.
Disequilibrium between pro-inflammatory and anti-inflammatory cytokine cascades is one of the mechanisms, along with telomere length, by which you might get the outcome of increased tissue healing at the expense of increased tendency towards cancer. I'm glad you pointed to Bret here. I immediately thought of his dissertation work when I read your title. I'm sure you know, but I'll point out that the same forces which caused this outcome in lab mice would be present in any laboratory animal, including these primates.
It would be interesting to know to what extent primates are compromised in this way. Since their lifespans are longer these evolutionary pressures have had fewer generations to affect them. Having said that, one could argue that the pressure would be stronger in primates. As primates are more expensive to maintain and have fewer children, there is a greater need to get as many offspring as possible from them while they're young. This is of course the same pattern that lead to the observed healing and cancer outcome in mice. My guess is that they wouldn't be as affected as mice, but that the economics of lab animals would be pushing them in a similar direction.
From my experience, the degree to which different control genotypes vary is shocking. I worked with drosophila as an undergraduate and several times had to run comparison experiments with flies from the same genotype from separate labs across the US. There were *always* significant differences between groups. This in a genetic lab that was very serious and thorough about the genetic background. Mouse labs are terrible with genetic controls in comparison. Unfortunately, this is only one of the major flaws undermining the foundation of scientific research.